Answer : As per the definition of the buffer solution "
It is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa".
so considering the above given condition for
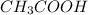 and
and
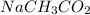 act as buffer because it satisfies and qualifies the conditions of behaving as a buffer when mixed in equal amounts,
act as buffer because it satisfies and qualifies the conditions of behaving as a buffer when mixed in equal amounts,
whereas HCl and NaOH are strong acid and strong base which does not satisfies the criteria for being called as buffer.
As buffer needs a weak acid and its conjugate base to behave and act as buffer and resist any pH change.