Answer 1) When a strong acid like
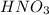
reacts with
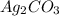
usually the equilibrium shifts to the right because
As per the Le chatelier's principle "if in any reaction, a dynamic equilibrium is disturbed by changing the any of the conditions, the position of equilibrium moves to counteract the change." So, in the given reaction when
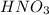
reacts with
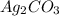
it generates carbon dioxide and water as a by product, if we are adding
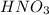
it will remove some of the
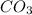
molecule from the reaction mixture, which then tends to shift the equilibrium towards right.
Answer 2) The same would be observed in this case, if we replace
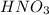
with HCl it will shift the equilibrium to the right as their will be generation of AgCl as the precipitate.
As per the definition of Le Chatelier's principle if we add reactants in the reaction the equilibrium will tend to move towards right, also if we replace the products or remove it then too it will shift the equilibrium towards right. So, in this reaction you are removing
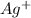
and
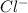
ions from the solution.