Answer :
87.60 g of Urea.
Explanation : We need to use the mole fraction formula here;
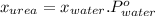
So, we have values and substituting them we get, 29.45 =
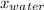
(31.8)
Hence,
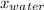
= 0.926 (this is the mole fraction of water)
Therefore, mole fraction of urea = 1 - 0.926 = 0.074,
moles of water present = 329 g / 18.0 mol = 18.27 moles,
Now, we have 0.074 moles of urea / 0.926 moles of water = x moles of urea / 18.27 moles of water,
Therefore, x = 1.46 moles of urea.
And now, Mass of urea = moles of urea X molar mass of urea;
= 1.46 X 60.0 =
87.60 g Hence the mass of urea to be dissolved in 329 g of water will be
87.60 g