Answer : The concentration of the resulting diluted solution will be 0.5 m.
Explanation : As we can see that the mole concentration is given as 2.00 m and the volume is 0.200 L and we need to find the moles in 0.800 L of the diluted solution.
Here the formula of
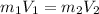
applies,
so on applying the formula we obtain the value of
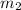
which is unknown in this case, will be :- (0.200 X 2.00) / 0.800 = 0.5 moles.