Answer : The volume of the resulting solution will be 0.4999 L.
Explanation : As the two molar concentration of the
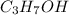
solution are given and one of the volume concentration needs to be found.
So, according to the formula :-
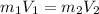
And here if we consider
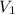
as 0.1718 L moles and
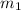
as 0.3556 moles then we need to find
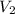
as
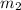
is given as 0.1222 moles.
Hence on solving the formula we get,
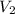
= (0.1718 X 0.3556) / 0.1222 = 0.4999 L