Answer : Option C)
6.3 X
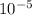 .
.
Explanation : Given is the concentration of benzoic acid is 0.200 M, now only one monoprotic acid ion is getting separated from it [
![[ H^(+) ]](https://img.qammunity.org/2019/formulas/chemistry/high-school/shdw9kxh8be3wf53rj3uqtqkx7ew9bsn88.png)
.
So, the reaction will be as,
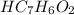
⇔
![[ H^(+) ]](https://img.qammunity.org/2019/formulas/chemistry/high-school/shdw9kxh8be3wf53rj3uqtqkx7ew9bsn88.png)
+
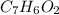
.
We can use the ICE method for calculation,
The molar concentration of
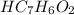
is 0.200 M and the [
![[ H^(+) ]](https://img.qammunity.org/2019/formulas/chemistry/high-school/shdw9kxh8be3wf53rj3uqtqkx7ew9bsn88.png)
is 3.55 X
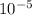
.
So here on reactant side we get, (0.2 - 3.55 X
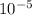
) = 0.196
on the product it remains as 3.55 X
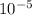
.
So,
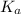
= (3.55 X
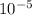
) X (3.55 X
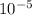
) / 0.196
We will get
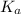
as
6.42 X
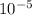 which is close to 6.3 X
which is close to 6.3 X
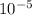 .
.