Answer : In the reaction of Ca +
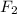
--->Ca
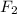
, the coefficient of Ca and
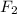
will be the same which will be 1.
Explanation : As per the rules of writing a chemical equation and balancing it properly, the number of elements in reactants side should be equal to the number of elements generated in product side. Hence when we use 1 molecule of Ca and one molecule of
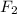
then it generates 1 molecule of Ca
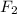
.