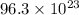Answer : The total number of atoms represented by the formula
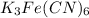 is,
is,
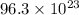
Explanation :
The given compound is,
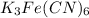
In the given compound, there are three moles of potassium (K) atoms, one moles of iron (Fe) atom, six moles of carbon (C) atoms and six moles of nitrogen (N) atoms.
Thus, the total moles of
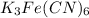 = 3 + 1 + 6 + 6 = 16
= 3 + 1 + 6 + 6 = 16
As we know that,
1 mole contains
 number of atoms.
number of atoms.
So, 16 moles contains
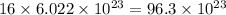 number of atoms.
number of atoms.
Therefore, the total number of atoms represented by the formula
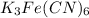 is,
is,