Answer : As given in the question the concentration of acid and base are in equimolar proportion so,
the simple net ionic reaction that the system is undergoing can be given as,

we can now calculate the total number of moles reacting in the species as,
(30 mL) X (1L/1000ML) X 0.150 moles =
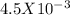
moles.
Now, we know the specific heat of water as 4.186 J/g °C.
Now considering the total volume of the product as 60 mL or 60 g (as it is water it can be g or mL) hence, ΔC is 2.5 C.
Now converting these into joules = 4.186 J/g °C X 2.5 X 60 = 627.9 Joules.
On dividing this we get 627.9 Joules /
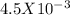
moles = 140 KiloJoules/mole.
So the answer is 140 KJ/M.