Hello!
What is the initial temperature of a gas if the volume changed from 1.00l to 1.10l and the final temperature was determined to be 255 ?
We have the following data:
V1 (initial volume) = 1.00 L
V2 (final volume) = 1.10 L
T1 (initial temperature) = ? (in Kelvin)
T2 (final temperature) = 255 K
According to the Law of Charles and Gay-Lussac in the study of gases, in an isobaric transformation, ie when a mass under pressure maintains its constant pressure, on the other hand, as the volume increases, the temperature increases and, if the volume decreases, the temperature decreases (directly proportional to temperature and volume) . We apply the data to the formula of isobaric transformation (Charles and Gay-Lussac), we will see:
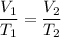
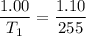
multiply the means by the extremes

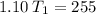
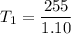

Answer:
The initial temperature is approximately 231.8 Kelvin
________________________