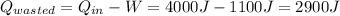(a) The efficiency of an engine is defined as the ratio between the work done by the engine and the heat it takes in:
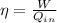
The engine in this problem does a work of
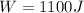
and it takes in
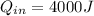
of heat, therefore its efficiency is
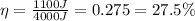
(b) The heat taken by the machine is 4000 J; of this amount of heat, only 1100 J are converted into useful work. This means that the rest of the heat is wasted. Therefore, the wasted heat is the difference between the heat in input and the work done by the engine: