Answer: The correct options are a, b and d.
Explanation:
Single displacement reaction is a type of chemical reaction in which a more reactive metal displaces a less reactive metal from its reaction. Equation for this reaction follows:
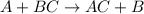
Metal A is more reactive than Metal B.
For the given options:
Option a: This reaction is a type of single displacement reaction because potassium is more reactive than lithium.
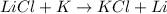
Option b: This reaction is a type of single displacement reaction because Magnesium is more reactive than Aluminium.
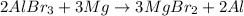
Option c: This reaction will not yield any product because copper is less reactive than lead.
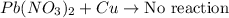
Option d: This reaction is a type of single displacement reaction because Sodium is more reactive than Calcium.
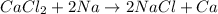
Hence, the correct options are a, b and d.