Answer:
4.65 L is the final volume of the gas.
Step-by-step explanation:
Initial pressure of the gas,
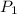 = 1.50 atm
= 1.50 atm
Initial volume of the gas,
 = 5.0 L
= 5.0 L
Final pressure of the gas ,
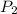 = 1240 mmHg = 1.612 atm
= 1240 mmHg = 1.612 atm
Final Volume of the gas =

Applying Boyle's law:
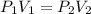 (Constant temperature)
(Constant temperature)
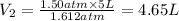
4.65 L is the final volume of the gas.