Answer: The final temperature of the gas is 7.58 °C.
Step-by-step explanation: We are given initial and final pressure of the system and we need to find the final temperature of the system.
To calculate it, we use the equation given by Gay-Lussac.
His law states that pressure is directly related to the temperature of the gas.
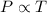
Or,
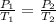
where,
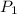 = initial pressure = 893 mmHg = 1.175atm (Conversion factor: 1atm = 760mmHg)
= initial pressure = 893 mmHg = 1.175atm (Conversion factor: 1atm = 760mmHg)
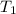 = initial temperature = 49.3°C = [49.3 + 273.15]K = 322.45K
= initial temperature = 49.3°C = [49.3 + 273.15]K = 322.45K
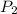 = Final pressure = 778mmHg = 1.023atm
= Final pressure = 778mmHg = 1.023atm
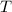 = Final temperature = ?°C
= Final temperature = ?°C
Putting values in above equation, we get:
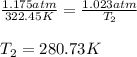
Converting Final temperature from kelvin to degree Celsius.
![T_2=280.73K=[280.73-273.15]^oC=7.58^oC](https://img.qammunity.org/2019/formulas/chemistry/high-school/f3dts794u561tfrv8394ljwv5l2tsmmwla.png)
Hence, the final temperature of the gas is 7.58 °C.