Answer: Molar mass of
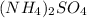 is 132.17 g/mol
is 132.17 g/mol
Step-by-step explanation:
Molar mass is defined as the mass of each element present in a 1 mole of a given compound. It is expressed in grams per mole (g/mol).
For calculating the molar mass of ammonium sulfate having chemical formula
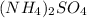 which contains 2 atoms of nitrogen, 8 atoms of hydrogen, 1 atom of sulfur and 4 atoms of oxygen.
which contains 2 atoms of nitrogen, 8 atoms of hydrogen, 1 atom of sulfur and 4 atoms of oxygen.
We are given:
Mass of Nitrogen = 14.01 g/mol
Mass of Hydrogen 1.01 g/mol
Mass of Oxygen = 16 g/mol
Mass of Sulfur = 32.07 g/mol
Calculating the molar mass of ammonium sulfate, we get:
![(NH_4)_2SO_4=[(2* 14.01)+(8* 1.01)+32.07+(4* 16)]g/mol=132.17g/mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/dngvh44azf1f0mjnj2otjk0fksls92upsp.png)
Hence, the molar mass of
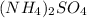 is 132.17 g/mol
is 132.17 g/mol