Answer: The more reactive element replaces lesser reactive one
Step-by-step explanation:
Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
A more reactive element is one which can easily lose or gain electrons as compared to the other element.
Example:
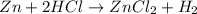
Here Zinc being more reactive than hydrogen can easily displace hydrogen from its salt solution and form zinc chloride and hydrogen in elemental form.