Answer: Option (d) is the correct answer.
Step-by-step explanation:
A chemical equation is defined as an equation in which symbolic representation of reactants is placed on left hand side and symbolic representation of products is placed on right hand side.
For example,
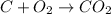
Here, C and
 are the symbolic formulas of carbon and oxygen atoms that shows reactants. Whereas
are the symbolic formulas of carbon and oxygen atoms that shows reactants. Whereas
 is the symbolic formula of carbon dioxide that shows products.
is the symbolic formula of carbon dioxide that shows products.
Thus, we can conclude that a symbolic representation of a chemical reaction based on the conservation of mass is an equation.