The choices are not given, however, the question can still be solved.
We know that:pH + pOH = 14
Converting the above equation to concentration form, we will get:[H₃O+] * [OH-] = 10⁻¹⁴
We are given that:concentration of hydroxide ion [OH-] = 1.4 * 10⁻¹⁰ M
Substitute with this in the above equation to get concentration of hydronium ion as follows:[H₃O+] * [OH-] = 10⁻¹⁴
[H₃O+] * [1.4 * 10⁻¹⁰ ] = 10⁻¹⁴
[H₃O+] =
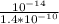
[H₃O+] = 7.1428 * 10⁻⁵ M
Hope this helps :)