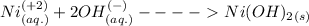Answer : When we combined the aqueous solutions of nickel nitrate and potassium hydroxide a displacement reaction will occur.
The whole reaction is explained below:-
![Ni(NO _(3)) _(2) + 2 KOH ----\ \textgreater \ Ni(OH) _(2) + 2 [KNO _(3) ].](https://img.qammunity.org/2019/formulas/chemistry/college/bks4wei1w3mvjhajwgxkyyj40pryssv4uf.png)
Now according to the OWL preparation page soluibility rules the compounds that are included in the reactants are perfectly soluble and hence undergo a displacement reaction yielding a precipitate at the completion of reaction.
The net ionic equation of the reaction is :