According to Coulomb's law, the larger the distance between the two charged particles, the weaker the force. In fact, the expression for the electrostatic force between two charged particles (called Coulomb's law) is given by
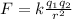
where
k is the Coulomb's constant
q1 and q2 are the two charges
r is their separation
We see that the force depends on the inverse of the square of the distance,
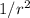
, therefore the larger the distance, the weaker the force.