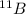Answer : The atom with the same number of neutrons as
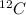 is,
is,
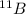
Explanation :
- The given atom is,
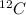
Atomic mass number = 12
Atomic number = 6
Atomic number = Number of protons = Number of electrons = 6
Number of neutrons = Atomic mass number - Atomic number = 12 - 6 = 6
Now we have to determine the number of neutrons in the given options.
- In atom,
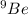
Atomic mass number = 9
Atomic number = 4
Atomic number = Number of protons = Number of electrons = 4
Number of neutrons = Atomic mass number - Atomic number = 9 - 4 = 5
- In atom,
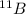
Atomic mass number = 11
Atomic number = 5
Atomic number = Number of protons = Number of electrons = 5
Number of neutrons = Atomic mass number - Atomic number = 11 - 5 = 6
- In atom,
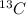
Atomic mass number = 13
Atomic number = 6
Atomic number = Number of protons = Number of electrons = 6
Number of neutrons = Atomic mass number - Atomic number = 13 - 6 = 7
- In atom,
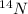
Atomic mass number = 14
Atomic number = 7
Atomic number = Number of protons = Number of electrons = 7
Number of neutrons = Atomic mass number - Atomic number = 14 - 7 = 7
Therefore, the atom with the same number of neutrons as
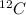 is,
is,