Answer:
The correct answer is 'potential to store heat'.
Step-by-step explanation:
Heat content is the amount of energy stored in the system both kinetic and potential. Each and every substance stores both energies.
The kinetic energy is due to motion of particles and potential energy is due to chemical or nuclear bond holding the system together.
In thermodynamics it is defined as sum of internal energy and product of pressure and volume.
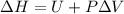
ΔH = heat content pf enthalpy of the system
PΔV = Work done by the system