Step-by-step explanation:
A chemical reaction in which a hydrocarbon reacts with oxygen atom and results in the formation of carbon dioxide and water is known as combustion reaction.
For example,
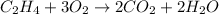
Here, ethene is the hydrocarbon which is reacting with oxygen atom and yields carbon dioxide and water.
Therefore, we can conclude that the given reaction is a combustion reaction.