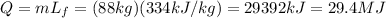During a change of state from liquid to solid (like in this problem, from liquid water to ice), the amount of heat that it is liberated is given by
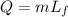
where
m is the mass of the substance
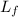
is the latent heat of fusion of the substance.
For ice, the latent heat of fusion is
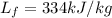
, while the mass of the water in this problem is m=88 kg. If we substitute these data into the equation, we find the amount of heat liberated: