Answer: Option (c) is the correct answer.
Step-by-step explanation:
An atom or specie that has the most negative
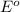 value will act as the good reducing agent, that is, the atom has lost the number of electrons. Thus, it itself gets oxidized.
value will act as the good reducing agent, that is, the atom has lost the number of electrons. Thus, it itself gets oxidized.
Whereas when an atom or specie has the most positive
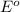 value will act as the good oxidizing agent, that is, the atom has gained the electrons. Thus, it itself gets reduced.
value will act as the good oxidizing agent, that is, the atom has gained the electrons. Thus, it itself gets reduced.
Hence, we can conclude that out of the given options Fe3+ + e- Fe2+ = 0.77 V is the half-reaction that is most easily reduced.