The formula for half-life is:
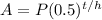
A is the amount remaining, P is the initial amount, t is the time that has passed, and h is the half-life of the substance. Plug in the values that you know and solve:
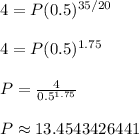
Rounded to the nearest whole number, the density of the substance was about 13
mg / cm².