Answer:
The final concentration is 0.01 M
Step-by-step explanation:
The Molarity (M) or Molar Concentration is the number of moles of solute that are dissolved in a given volume.
Molarity is then expressed as:
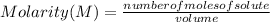
Molarity is expressed in units
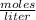
To know the concentration in 200 mL you must first know the amount of moles. This is the same amount of moles you have initially, and is calculated by:
Molarity*Volume=number of moles
0.04 M* 0.05 L =number of moles , where 0.05 L= 50 mL (1 L=1000 mL)
2*10⁻³ = number of moles
It is now possible to calculate the molarity in a volume of 200 mL = 0.2 L:
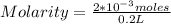
Molarity = 0.01 M
The final concentration is 0.01 M