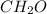Answer:
Empirical formula of the compound =
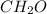
Step-by-step explanation:
Empirical formula is the mole ratio of each element in a chemical compound.
Given:
No. of mol of C = 0.100 mol
No. of mol of H = 0.200
No. of mol of O =
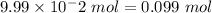
For the determination of empirical formula, no. of moles of each element should be in whole number.
If the no. of moles of elements are not in whole number, then divide moles of each element by the smallest mole of the element.
So, ratio of each element is:
C =
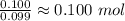
H =
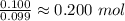
O =
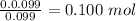
Mole ratio is
C : H : O = 1 : 2 : 1
So, the empirical formula =