To get the number of gold atoms, you have to divide the mass of the gold by the mass of the gold atom. It follows this simple equation
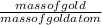
.
Let x be the number of gold atoms. Plug in the values to a calculator.
x =
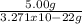
Both have the same units so the unit gram(g) can be cancelled.
x then would be equal to 1.53x10^22. So there are 1.53x10^22 atoms of gold in 5 g of gold