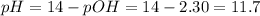Answer:
pH = 11.7
Step-by-step explanation:
Given:
[OH-] = 0.00500 M
To determine:
pH of the solution
Step-by-step explanation:
pH refers to the H+ ion concentration in a given solution whereas pOH indicates the OH- ion concentration.
pH and pOH are related by the following equation:
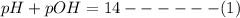
Here:
![pH = -log[H+]\\\\pOH = -log[OH-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/y0t0hwdp838a83b77u1tt8xme45qvfvvsk.png)
When OH- = 0.00500 M
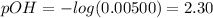
Based on equation(1):