Relative atomic mass for two isotopes can be calculated as follows
Isotope atomic mass abundance²⁰³Tl 203 29.5%
²⁰⁵Tl 205 70.5%
Relative atomic mass
= (mass of 1 isotope x % abundance) + (mass of 2 isotope x % abundance)
= (203 x
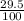
) + (205 x
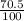
)
=
204.41 amu
Hence, relative atomic mass of Tl is 204.41amu