Answer:
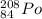
B is correct.
Step-by-step explanation:
Given the equation
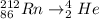
According to alpha emission
Alpha emission is a radioactive decay in which the atomic nucleus emit the alpha particle and formed a new nucleus whose mass number reduced by four and atomic number reduced by two.
Therefore,
Po is the new nucleus.
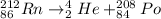
Where, Rn = Radon
Po= polonium