Answer: Option (D) is the correct answer.
Step-by-step explanation:
As the atomic number of magnesium is 12 and its electronic distribution is 2, 8, 2. Hence, in order to attain stability magnesium will readily lose its valence electrons.
Therefore, magnesium will form a
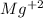 ion.
ion.
Whereas atomic number of chlorine is 17 and its electronic distribution is 2, 8, 7. And, in order to attain stability it will readily accept an electron from a donor atom.
Hence, on gaining one electron a neutral atom of Cl will convert into
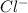 ion.
ion.
Therefore, magnesium chloride (
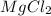 ) is the compound formed when both magnesium and chlorine chemically react with each other.
) is the compound formed when both magnesium and chlorine chemically react with each other.
Thus, we can conclude that the statement Mg atom donates one electron to each of the two Cl atoms, correctly explains how magnesium and chlorine combine to form magnesium chloride.