Step-by-step explanation:
An electrochemical cell is a cell in which chemical reactions take place to generate electricity. This is done with the help of oxidation and reduction-half reactions taking place at anode and cathode electrodes.
As a result, a cell potential generates and values of this cell potential can be measured as follows.
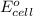 =
=
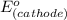 -
-
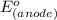
Thus, in an electrochemical cell, the cell potential is the measure of the reduction potentials of the two half-cells.