Answer: The correct answer is Option C.
Step-by-step explanation:
Oxidation state is defined as the number which is given to an atom when it looses or gains electron. It is written as a superscript.
When an atom looses electron, it will attain a positive oxidation state and when an atom gains electron, it will attain a negative oxidation state.
An atom looses or gains electron to attain stability and to fill their valence shell.
For Example:
- Oxygen atom will gain 2 electrons and will form
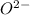 ion having oxidation state of -2.
ion having oxidation state of -2. - Sodium atom will loose 1 electron and form
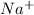 ion having oxidation state of +1.
ion having oxidation state of +1.
Thus, the correct answer is Option C.