We can solve the problem by using the first law of thermodynamics:
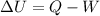
where
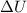
is the variation of internal energy of the system
Q is the heat absorbed by the system
W is the work done by the system
In this problem, the heat absorbed by the system is Q=+44 J, and the increase in internal energy is
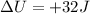
. So we can rearrange the equation to calculate the work done:
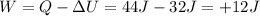
and the work is positive, which means that it is done by the system on the surrounding.