Hello!
In a schools laboratory students require 50.0 mL of 2.50 H2SO4 for an experiment but only available stock solution of the acid has a concentration of 18.0m. What volume of the stock would they use to make the required solution
0.900 mL
1.11 mL
6.94 mL
7.20 mL
We have the following data:
M1 (initial molarity) = 2.50 M (or mol/L)
V1 (initial volume) = 50.0 mL → 0.05 L
M2 (final molarity) = 18.0 M (or mol/L)
V2 (final volume) = ? (in mL)
Let's use the formula of dilution and molarity, so we have:
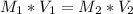
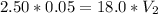
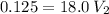
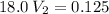
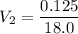
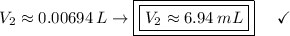
Answer:
The volume is approximately 6.94 mL
_______________________________