Answer:
23.459 g NaNO₂
General Formulas and Concepts:
Math
Pre-Algebra
Order of Operations: BPEMDAS
- Brackets
- Parenthesis
- Exponents
- Multiplication
- Division
- Addition
- Subtraction
Chemistry
Stoichiometry
- Reading a Periodic Table
- Using Dimensional Analysis
Step-by-step explanation:
Step 1: Define
[RxN] H₂SO₄ + 2NaNO₂ → 2HNO₂ + Na₂SO₄
[Given] 24.14714 g Na₂SO₄
Step 2: Identify Conversions
[RxN] 1 mol Na₂SO₄ = 2 mol NaNO₂
Molar Mass of Na - 22.99 g/mol
Molar Mass of N - 14.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of S - 32.07 g/mol
Molar Mass of Na₂SO₄ - 2(22.99) + 32.07 + 4(16.00) = 142.05 g/mol
Molar Mass of NaNO₂ - 22.99 + 14.01 + 2(16.00) = 69.00 g/mol
Step 3: Stoichiometry
- Set up:
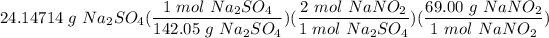
- Multiply/Divide:
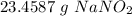
Step 4: Check
Follow sig fig rules and round. We need 5 sig figs (instructed).
23.4587 g NaNO₂ ≈ 23.459 g NaNO₂