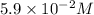Answer : The correct option is,
Solution : Given,
Equilibrium constant = 3.90
Concentration of
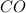 = 0.30 M
= 0.30 M
Concentration of
 = 0.10 M
= 0.10 M
Concentration of
 = 0.020 M
= 0.020 M
The balanced equilibrium reaction will be,
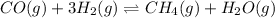
The expression for equilibrium reaction will be,
![K_p=([H_2O]* [CH_4])/([CO]* [H_2]^3)](https://img.qammunity.org/2019/formulas/chemistry/high-school/eunlqr46tgtrx7byyp113n59kszhuss8kw.png)
Now put all the given values in this expression, we get the concentration of methane.
![3.90=((0.020)* [CH_4])/((0.30)* (0.10)^3)](https://img.qammunity.org/2019/formulas/chemistry/high-school/m4mfjw1bexuwenlm42ikcu7tuu38shyoq6.png)
![[CH_4]=0.059=5.9* 10^(-2)M](https://img.qammunity.org/2019/formulas/chemistry/high-school/foe6n39pnh9i0cg0ki5lttc0zogeybc6yu.png)
Scientific notation : It is the way of representation or writing the very large and the small number. In scientific notation, a number is written when a number between 1 and 10 is multiplied by the power of 10.
Therefore, the equilibrium concentration of methane
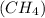 is,
is,