Step OneFind the moles of HCl
C = 1.0 mol/L
V = 75 mL = 75 / 1000 = 0.75 L
Note the concentration is in Liters.
Formulan = C*V
Solven = 1.0 * 0.075
n = 0.075 moles
Step two Adjust the number of moles of HCl
How every mol of H2 produced, you will require 2 mol of HCl
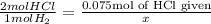
2/1 = 0.075/x Cross multiply
2x = 0.075 Divide by 2
x = 0.075 / 2
x = 0.0375
moles of H2 <<<<<<<<< Answer