Answer: must be nonpolar
Step-by-step explanation:
A polar covalent bond is defined as the bond which is formed when there is a difference of electronegativities between the atoms. It is also defined as the bond which is formed due to the unequal sharing of electrons between the atoms. Example:
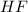
Non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms. It is also defined as the bond which is formed due to the equal sharing of electrons between the atoms. example:
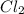
Thus as
 ,
,
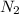 ,
,
 molecules are formed by similar atoms with no electronegativity difference, the bond must be non polar.
molecules are formed by similar atoms with no electronegativity difference, the bond must be non polar.
Hence, the correct answer is the molecules
 ,
,
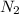 ,
,
 must be non polar.
must be non polar.