Answer: The average atomic mass of carbon is 12.09 amu
Step-by-step explanation:
Solution : Given,
Mass of isotope C-12 = 12 amu
% abundance of isotope C-12= 98.93% = 0.9893
Mass of isotope C-13 = 13.003 amu
% abundance of isotope C-13 = 1.07% = 0.0107
Mass of isotope C-14= 14.003 amu
% abundance of isotope C-14 = 0.0000000001% =
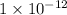
Formula used for average atomic mass of an element :
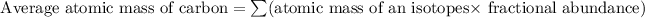
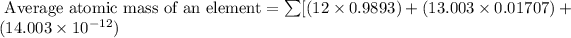
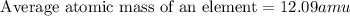
Therefore, the average atomic mass of carbon is 12.09 amu.