Answer:
1.10 g H₂
Step-by-step explanation:
The reaction is
2HCl + Mg → MgCl₂ + H₂
An excess of magnesium metal means that the limiting reactant is HCl, so we use the mass of HCl given by the problem to calculate how many grams of H₂ are produced.
In order to make this calculation we need to keep in mind the molar mass of HCl, of H₂, and the reaction stoichiometry:
40.0 g HCl *
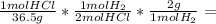 1.10 g H₂
1.10 g H₂