Answer: The given statement is false.
Step-by-step explanation:
Combination reactions are defined as the reactions in which two or more substances that can be either elements or compounds when combine together then they result in the formation of a single compound.
For example,
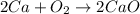
Also, when oxygen atom combines with another substance then it is known as a combustion reaction. And, a combustion reaction will always release energy as they are exothermic in nature.
But is not necessary that light will always be produced in a combustion reaction.
Hence, we can conclude that the statement oxygen is a reactant in a combination reaction, which always produces energy in the form of heat and light, is false.