Answer:
The mass of 21.05 moles of iron(III) sulfide is 4,372.085 grams.
Step-by-step explanation:
Iron (III) sulfide is a chemical compound with the formula Fe₂S₃.
Being the molar mass of the elements:
- Fe: 55.85 g/mole
- S: 32 g/mole
then the molar mass of iron sulfide (III) is:
Fe₂S₃= 2* 55.85 g/mole + 3* 32 g/mole= 207.7 g/mole
Molar mass is the amount of mass that a substance contains in one mole. Then the rule of three can be applied: if in 1 mole of iron (III) sulfide there are 207.7 grams, in 21.05 moles how much mass is there?
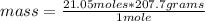
mass= 4,372.085 grams
The mass of 21.05 moles of iron(III) sulfide is 4,372.085 grams.