Answer: The product of the radioactive decay
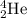 is called as alpha particle.
is called as alpha particle.
Step-by-step explanation:
Radioactive decay of the nuclei happens when a nuclei has to attain some sort of stability. It undergoes various decay processes and releases various particles during these decay process. The types of particles released are:
- Alpha particle: These are released during alpha decay. The particle released carry a mass of +4 units and a charge of +2 units. It is represented as
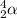
- Beta particle: These are released during beta decay. The particle released carry a mass of 0 units and a charge of -1 units. It is represented as
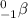
- Gamma particle: These are released during gamma decay. The particle released does not carry any charge and is electrically neutral. It is represented by
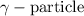
Hence, the correct answer is alpha particle.