Answer:
The correct answer is option (a).
Step-by-step explanation:
Suppose we have total mass of the mixture be 100 g
let the mass of KCl be x
let the mass of
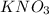 be y
be y
x + y = 100...(1)
Moles of KCl =
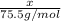
Moles of K in KCl will be =
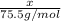 (1 mol is present in 1 mole of molecule)
(1 mol is present in 1 mole of molecule)
Moles of
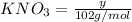
Moles of K in
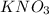 will be =
will be =
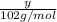 (1 mol is present in 1 mole of molecule)
(1 mol is present in 1 mole of molecule)
Percentage of potassium in the mixture = 44.20 %
In 100 g, the mass of potassium = 44.20 g
Moles of potassium =
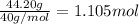
So,
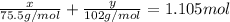 ..(2)
..(2)
Solving equation (1) and (2):
we get ,y = 63.78 g, x=36.22 g
Percentage of KCl:
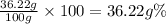
The percent of KCl in the mixture is closest to 40%.Hence ,option (a) is correct.