Answer : The molecular weight of
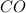 is, 28.01 g/mole
is, 28.01 g/mole
Explanation : Given,
Weight of one mole of carbon = 12.01 g
or, Atomic weight of carbon = 12.01 g/mole
Weight of one mole of oxygen = 16.00 g
or, Atomic weight of oxygen = 16.00 g/mole
Molecular weight : It is defined as the sum of the atomic weight of the element present in the molecule.
The molecular weight of CO = Atomic weight of carbon + Atomic weight of oxygen
The molecular weight of CO = 12.01 g/mole + 16.00 g/mole
The molecular weight of CO = 28.01 g/mole
Therefore, the molecular weight of
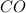 is, 28.01 g/mole
is, 28.01 g/mole