Answer: 25% will be remaining.
Explanation: Half live is the time in which the a radioactive substance remains half of its initial amount.
Given half life is 5200 years. let's say we have 100 grams(assume any amount of the element). In one half life(5200 years), half of it's initial amount that is 50 grams will be remaining. Now, in second half life, 25 grams of the element will be remaining.
Percentage of the remaining element =
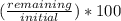
percentage of the remaining element =
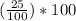 = 25%
= 25%
So, 25% of the element will be remaining in two half lives.