First of all, let's find the number of moles of the gas.
The molar mass of argon is

. Since we have

of gas, the number of moles is

Now we can use the ideal gas law to calculate the pressure of the gas:
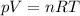
where
p is the pressure

is the volume

is the number of moles

is the gas constant

is the absolute temperature
Rearranging the equation, we find
